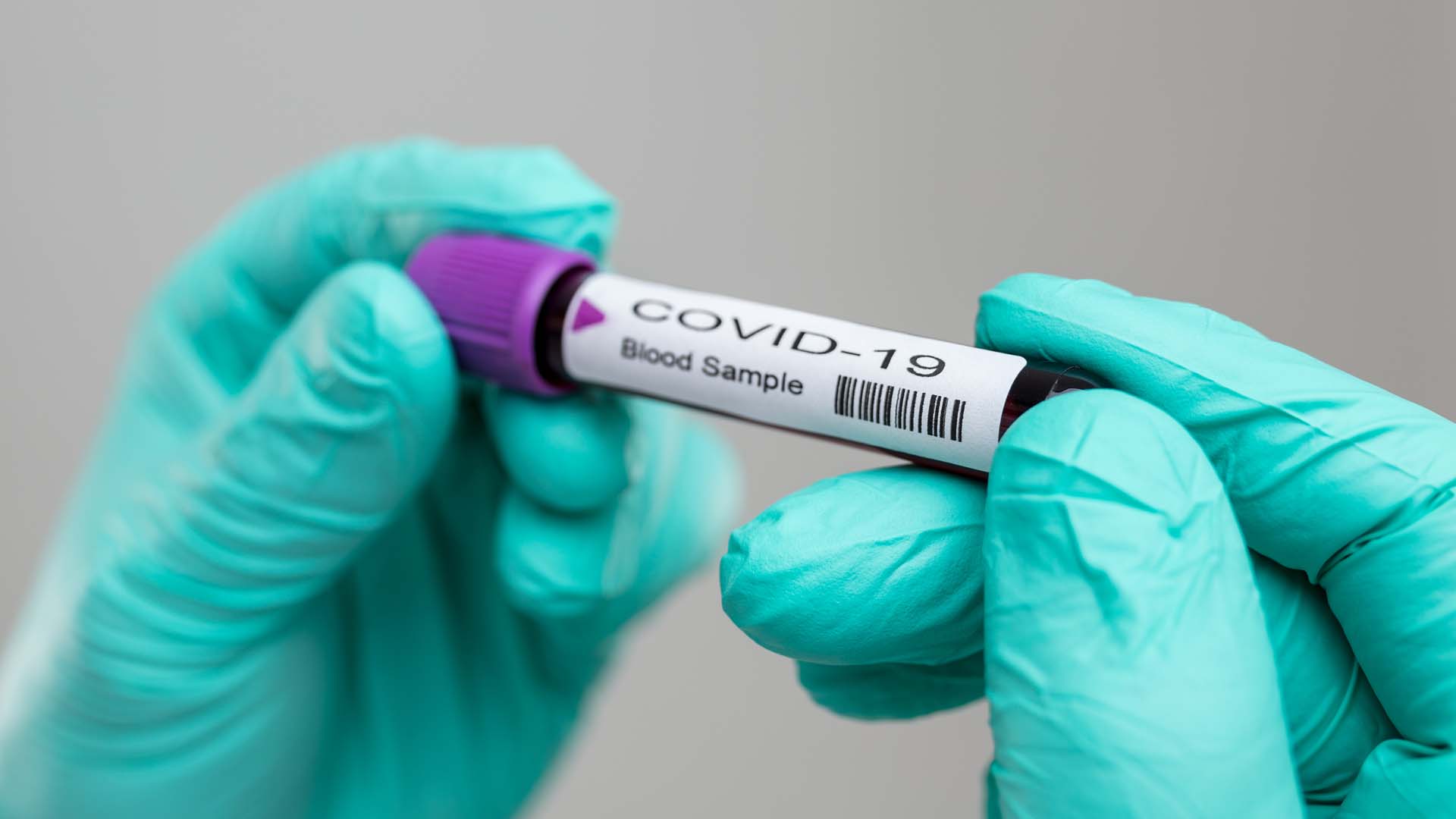
The University of the Philippines-National Institutes of Health (UP-NIH) is targeting 16,000 tests for coronavirus disease 2019 (Covid-19) per day by May 1 as its locally-produced kits will be offered at its facility starting tomorrow.
During the Beat Covid-19 virtual presser on Sunday, UP-NIH biotechnology researcher and founder and CEO of Manila Healthtek Inc., Dr. Raul Destura, said they are currently manufacturing 6,000 to 8,000 tests per day.
“So when we announce we don’t use the word ‘kit’ because it is very confusing. We use the word test so we know that one test (is) one person. When we say, our manufacturing capacity is 6,000, 8,000 tests that is 6,000 (to) 8,000 people a day,” he said.
Test kits
Destura said the locally-produced kits are Real-Time Polymerase Chain Reaction (RT-PCR) kits which can perform ‘one-step tests’ — identification of the target in a single run with internal control for monitoring the technologist’s performance.
“Ang ginawa namin ay (What we created is) based on our capacity to run to avoid the kit getting broken, one kit is 25 reactions, that is also the maximum allowable, physiologically, of one medical technologist before a problem with the result arises. We created a batch of 25 so you just take out one from the refrigerator, to protect the kits,” he said.
He added that the kits have undergone two testing pathways.
“‘Yung analytical performance tinest namin siya sa lahat ng pwedeng bacteria and virus na nasa baga, dapat hindi nagpa-positive except sa coronavirus lang, at the same (time) tinest namin siya kung gaano kababa na virus ang kaya niyang i-pick-up which is 0.27 copies per one microlitre, very small pwede niyang ma-amplify (On the analytical performance, we tested the kit in all possible bacteria and virus in the lungs. It shouldn’t be positive except for coronavirus only, and at the same time we tested how the smallest virus it can pick up which is is 0.27 copies per one microlitre, it’s very small and the kit can amplify it),” he said.
The kits also underwent validation study by a third party before UP-NIH was given the authority to market them on April 3.
“The kit is already a full set as it has Covid-19 testing kit partnered with extraction kit, and with viral transfer medium plus the two swabs — nasopharyngeal and oropharyngeal swabs. There will be no lags…re-agents, extraction and transfer mediums are all included,” Destura said.
Citing that other RT-PCR test kits could provide “very sensitive numbers and very specific numbers” based on laboratory performance data, Destura said field validation, which was performed on the locally-produced test kits, takes into account the skill set of the one performing the test and how long the sample was stored.
“That’s the best part our government did. We had local field validation, something we can be proud of actually.
Training
Destura emphasized that the test kits will only be sold to the laboratories certified capable of performing tests on specimens from Covid-19 patients by the DOH.
Moreover, laboratories that will buy the test kits are required to have their personnel trained by the UP-NIH.
“Due to physical distancing, we developed teaching videos so they can study in advance, so when the team arrives, one day or half-a-day post running then we’ll give them proficiency tools just to make sure if they get how it works. These laboratory personnel are used to handling biological samples, what must be added are the skill sets on proper laboratory practices on doing PCR at the same time proper laboratory behavior on biological safety because we don’t want to have laboratory-acquired infections,” Destura said.
The training may take one to one and a half days depending on the skills of the laboratory personnel, whether it is their first time or if they are used to performing such tests.
“They will have to do the whole process of return demo to show what they’ve learned. There will be 24 hours technical support, for example, they find it difficult to read the results with the software, we’ll do online tutoring with the pathologist so they will be empowered because after a while they will get use to it,” Destura said.
He added that such training is advantageous for all local government units (LGUs).
“This is already bio-preparedness because epidemics always happen in this world. That’s the good thing about this program because we’re empowering the LGUs to respond better the next time,” he said. (PNA)